Open Software.
100% Free.
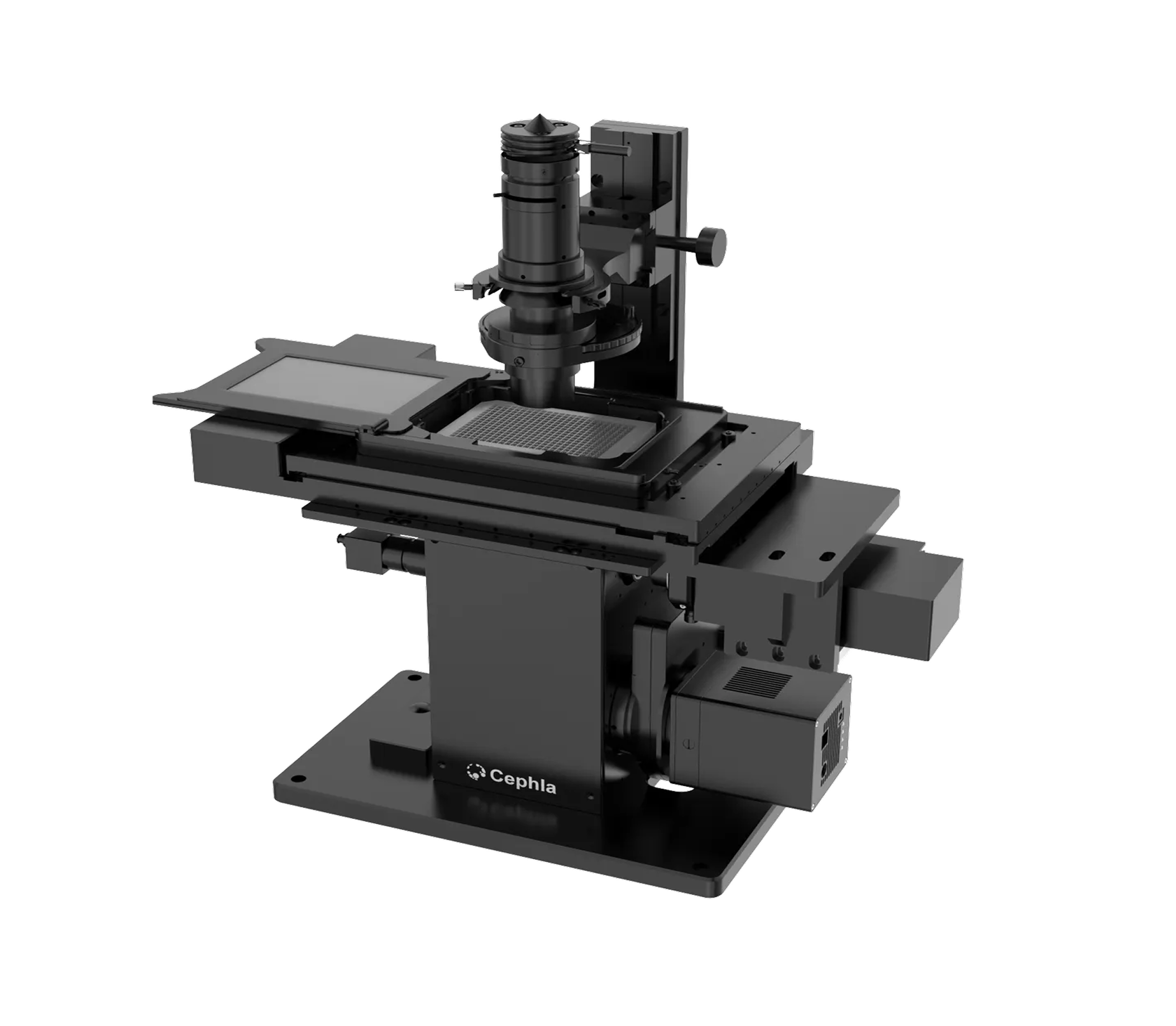
Control our hardware and third-party tools —
from cameras to robots — for fully automated imaging

Andrew Lu
from Elowitz Lab, Caltech
"I have used quite a lot of different imaging softwares in the past year. Yours was really excellent and easy to use. Just enough detail and visually easy to navigate."